Surgical Implants: Have You Considered ALL Aspects of Safety & Compliance?
How Confident Are You?
November 2023
By Jody Randall MSN, RN, CIC, HACP-CMS, HACP-PE
CEO and Founder
The use of surgical implants in a hospital setting is a critical and highly regulated aspect of patient care. Hospitals typically have specific guidance and protocols in place to ensure the safe and effective use of surgical implants. Below are some key considerations that hospitals may include in their guidance for the use of surgical implants:
- Regulatory Compliance :
Hospitals must comply with local, national, and international regulations and standards governing the use of surgical implants. This includes adhering to guidelines set forth by regulatory bodies such as the FDA (in the United States), the European Medicines Agency (EMA), or other relevant authorities.
- Product Selection and Approval: Hospitals should establish procedures for evaluating and approving surgical implant products for use. This includes vetting suppliers and manufacturers, assessing the quality and safety of implants, and ensuring compliance with regulatory requirements.
- Credentialing and Privileging:
Hospitals should have a process for credentialing and privileging surgeons and healthcare professionals who are qualified to implant devices. This process ensures that healthcare providers have the appropriate training and experience.
- Informed Consent:
Hospitals must ensure that patients receive comprehensive and understandable information about the surgical implant procedure, including its risks and benefits. Informed consent is a critical step in the process.
- Patient Assessment:
A thorough assessment of the patient's medical history, condition, and suitability for implantation is necessary. This includes evaluating the patient's overall health, potential allergies, and any contraindications for the specific implant.
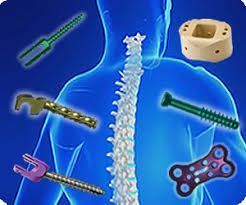
- Surgical Procedures:
Hospitals should have established surgical protocols for the implantation of devices, including pre-operative, intra-operative, and post-operative procedures. These should ensure aseptic techniques, patient safety, and quality care.
- Implant Tracking and Documentation:
Proper record-keeping and tracking of implantable devices are essential. This includes maintaining detailed records of the type of implant, its lot number, and its manufacturer. In case of product recalls or adverse events, accurate tracking can be crucial.
- Surveillance and Monitoring:
Hospitals must have systems in place for monitoring the performance and outcomes of implant procedures. This can include post-operative follow-up, tracking complications or adverse events, and ensuring the long-term effectiveness of the implant.
- Infection Control and Sterilization:
Strict infection control and sterilization protocols are critical to minimize the risk of surgical site infections and other complications associated with implantation.
- Staff Training and Education:
Hospitals should provide ongoing training and education for healthcare professionals involved in the implantation process. This ensures that staff members are aware of the latest techniques, safety measures, and best practices.
- Adverse Event Reporting:
A process for reporting and addressing adverse events related to surgical implants should be in place. Hospitals should follow established procedures for reporting adverse events to the appropriate regulatory agencies and manufacturers.
- Patient Follow-Up and Postoperative Care:
Comprehensive postoperative care and follow-up protocols are essential to monitor patient recovery, assess the performance of the implant, and address any issues that may arise.
- Quality Assurance and Continuous Improvement:
Hospitals should have mechanisms for ongoing quality assurance and continuous improvement in the use of surgical implants. This includes regular reviews of implant procedures, outcomes, and feedback mechanisms to identify areas for improvement.
The guidance for the use of surgical implants in a hospital will vary depending on the specific hospital, location, and the types of surgical procedures performed. Hospitals typically collaborate with healthcare providers, regulatory agencies, and professional organizations to develop and refine their implantation protocols and procedures.
In our experience, the biggest challenges surround lack of knowledge. Employees and providers may not fully understand regulatory requirements for using surgical implants. It is important to ask surgical device representatives for all information about products, related research, and FDA approval status in order to make informed decisions about possible risk and safety concerns associated with a product.
Tracking of implants is another critical element that may not be fully understood but documentation is critical in the event there is a product recall. Credentialing is another area that is easily overlooked. Have providers been granted privileges to implant surgical devices?
There is no better time than now to do a deep dive in to your organization’s current practices regarding surgical implants.
Use of surgical implants is one of the most complex topics in healthcare today. There is a high rate of failure associated with meeting regulatory requirements compliance due to an extensive list of criteria that should be considered to ensure that use of surgical implants is safe for patients.
When in doubt, call the experts for help! HCE is Here
Healthcare Consulting Experts LLC was built based upon our understanding of the challenges that healthcare facilities are facing today. Healthcare professionals strive to deliver the best possible care to all patients. We can help your facility through the difficult times and put you back on track to a less stressful tomorrow.
Don’t take chances! Our experts can assist with regulatory compliance requirements for whether you are building a new, state-of-the-art project or renovating an existing structure. Be sure to visit
Our Website
to see a full list of the services that we provide.
Contact us today at +1 (800) 813-7117 for a free initial consultation.
Please join us by clicking on any of our icons below to leave a comment or for more information and updates