Point-of-Care Testing ... Challenges For Your Facility
Considerations For Performing POCT Correctly
august 2022
By Jody Randall MSN, RN, CIC, HACP-CMS, HACP-PE
CEO and Founder
Many organizations have integrated Point-of-Care Testing (POCT) into their daily operations. You will find POCT technology used in medical offices as well as acute care settings. Testing devices are typically small machines that can be transported by hand or easily moved around patient care locations on small carts. There are a variety of laboratory tests that can be performed by healthcare personnel at the bedside.
Advantages
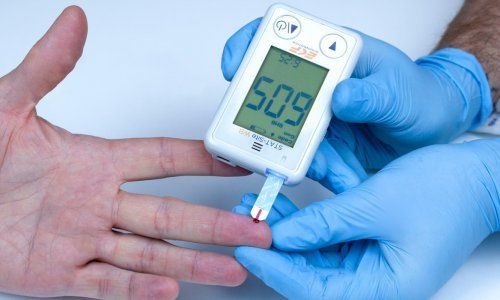
One of the biggest advantages to point of care testing is that it offers immediate results that can alert care providers to critical clinical events. One of the most commonly used POCT devices is the glucometer. These are also very commonly used by diabetic patients at-home to offer immediate results. Another example of POCT is the iSTAT. This handheld machine provides instant results for blood gas, cardiac enzyme, and coagulation levels. This type of technology can assist health care providers with early results enabling them to provide rapid treatment for emergent medical conditions. Another point- of-care test commonly used in healthcare settings is electrocardiogram also known as ECG or EKG. Once conduction leads are placed on a patient and cables are connected, a comprehensive analysis of cardiac rate and rhythm can be obtained in seconds. This type of analysis is a key component of a cardiac work up in every emergency room today.
Another advantage for POCT is the convenience for both the patient and healthcare personnel. POCT can provide diagnostic testing at a reduced cost. Testing performed may be less invasive for patients. Immediate results can provide information that may assist treating providers with early identification of a change in a patient’s condition.
Disadvantages
It is important to understand to risks that can be associated with POCT. One of the areas that our consultants find deficiencies is with staff competency. Healthcare organizations must be able to demonstrate that healthcare personnel have been properly trained to perform POCT. Competency should include key elements such are quality control testing, proper patient identification, unique user identification, aseptic technique and use of appropriate personnel protective equipment depending on the type of testing being performed. Competency should also include a return demonstration of all steps to the qualified trainer. Employers must be able to demonstrate proof of competency for trained personnel who perform POCT. Competency testing should be done for all new team members performing testing and no less than annually.
All equipment used should be properly maintained according to the manufacturer’s recommendations. A log should be kept providing evidence that required maintenance in being performed. In larger healthcare facilities, the Bio-Medical technician oversees servicing and maintenance of this type of equipment and maintains records and log of preventative, routine maintenance, and service for all medical equipment in use and out of service.
Many of the POCT machines that are used at the bedside also require additional quality assurance (QA) measures. For example, a glucometer may require a QA test to be done on the machine every twenty-four hours. This often includes performing a test using reagents that will produce results that mimic a high or low blood sugar result. If not performed, the end user will likely discover that the machine is locked and they are not able to use the machine until a proper QA test is performed. ECG machines typically have a limited storage capacity and patient information should be properly secured or backed up in order not to lose critical information.
Other considerations include how results are being recorded into the patient’s medical records if results are obtained at the bedside. Ideally this type of equipment will store results which are then uploaded safely and securely into the electronic medical records system before this information is accidently deleted.
Like with all technology, equipment malfunction can be a very serious reality. The Manufacturer and User Facility Device Experience (MAUDE) database is maintained by the Food and Drug Administration (FDA). It is a database wherein providers can report instances of equipment malfunction associated with adverse outcomes or death. This website is intended to encourage reporting of adverse events related to medical equipment or devices. There are cases in which POCT equipment did not provide accurate test results when validated by traditional venous or arterial blood testing methods. In many cases patients were incorrectly diagnosed and treated as a result. The National Institutes of Health referenced
this article from the National Library of Medicine
detailing the evolution of POCT.
In summary, POCT is certainly not going away but will continue to be essential in the fast-paced, demanding healthcare climate of today. We at HCE would like to remind healthcare providers to consider all advantages and disadvantages associated with POCT. Be sure you enlist the support of qualified personnel to oversee maintenance of medical equipment in your organization. We cannot stress enough how important it is to have a thorough training program for healthcare personnel who will perform POCT and to be able to demonstrate these individuals are competent to perform these duties. It is critical to have well written policies and procedures outlining critical results reporting and validation of test results. Finally, documentation and reporting of adverse events is important to not only notify manufacturers of equipment malfunction but also prevent future instances by reporting such matters. For guidance regarding reporting these events,
click here
for more information.
Contact HCE today to see how our experts can assist you with ensuring that your organization is providing safe and compliant POCT. Call us today at 1(800)813-7117 for a free consultation. We are here for you!
References
https://www.fda.gov/regulatory-information/search-fda-guidance-documents/questions-and-answers-about-emdr-electronic-medical-device-reporting-guidance-industry-user
HCE is Here to Help
Healthcare Consulting Experts LLC was built based upon our understanding of the challenges that all healthcare facilities are facing today. Healthcare professionals strive to deliver the best possible care to all patients. We can help your facility through the difficult times and put you back on track to a less stressful tomorrow.
Don’t take chances! Our experts can assist with regulatory compliance requirements for whether you are building a new, state of the art project or renovating an existing structure. Be sure to visit our website at
www.healthcareconsultingexperts.com
to see a full list of the services that we provide. Contact us today at 1(800) 813-7117 for a free initial consultation.
Please join us by clicking on any of the icons below to leave a comment or for more informati
on and updates:





